Purified Water Analysis
Chemical and Microbiological Tests
What is Purified Water?
Purified Water is the number one raw material used in pharmaceutical manufacturing. It is used at almost every stage of the manufacturing process from washing and cleaning to the primary ingredient in a final non-intravenous product formulation. It is produced through a multi-stage purification process usually including ion exchange and reverse osmosis. Due to the critical nature of water and water systems, Purified Water Testing is a regulatory requirement to perform routine microbiological and chemical quality assessments.
Why and how do we test it?
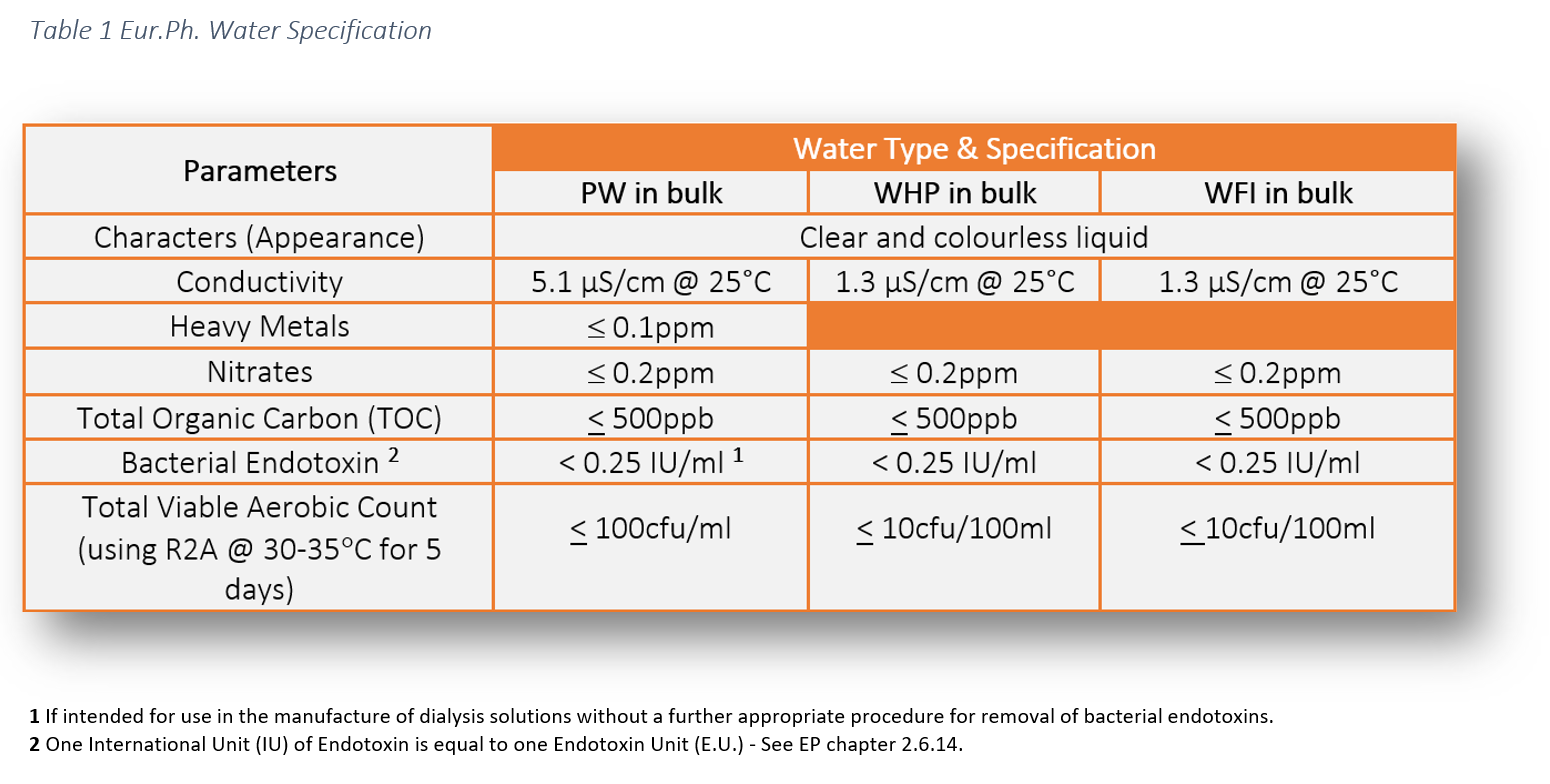
The legal requirement to comply with pharmaceutical water specifications is defined in the pharmacopoeial monographs. These water specifications can be found in the following pharmacopoeias: USP, Ph. Eur. and JP. This literature establishes the minimum quality standards within the industry fro Purified Water Testing, which are enforceable by the regulators. As manufacturers and service providers we have a responsibility to ensure the highest level of operational control to ensure product safety for patients.

How can we help assure compliance?
At Honeyman we are able to provide the full suite of microbiological and chemical testing to ensure compliance with the following:
- European Pharmacopoeia (Ph. Eur.)
- United States Pharmacopoeia (USP)
- Harmonized tests covering both Ph. Eur. and USP (Ph. Eur./USP/JP)
- Japanese Pharmacopoeia (JP)
- In-house specifications
To satisfy the microbiological quality component we are able to perform:
- Total viable count (TVC) via membrane filtration
- Total viable count (TVC) via pour plate
Our suite of chemical tests to ensure compliance include:
- Conductivity
- Nitrates
- Total Organic Carbon (TOC)
- Heavy Metals
We can also undertake client-specific analysis to in-house specifications, for example pH & endotoxin.
Purified Water Analysis
Contact Us To Request a Quotation or Book a Test:
Our Customers: