Water Highly Purified (WHP)
Detecting Endotoxins with TVC and LAL Assay
What is Water Highly Purified (WHP)?
Purified Water is the number one raw material used in Pharmaceutical manufacturing. WHP is a further purification of pharmaceutical Purified Water via two-stage reverse osmosis (RO), this RO step removes bacterial endotoxins from the water. Prior to the changes to the pharmacopoeia in April 2017, where it defines the acceptance of WFI produced by RO, WHP was a bridge between PW and WFI. Implementing this intermediate quality of water allowed industry data surrounding the effectiveness of RO to produce WFI to be gathered. Recent changes mean this grade of water is relatively rare in the industry with most people electing for distillation to produce WFI.
Why do we test it?
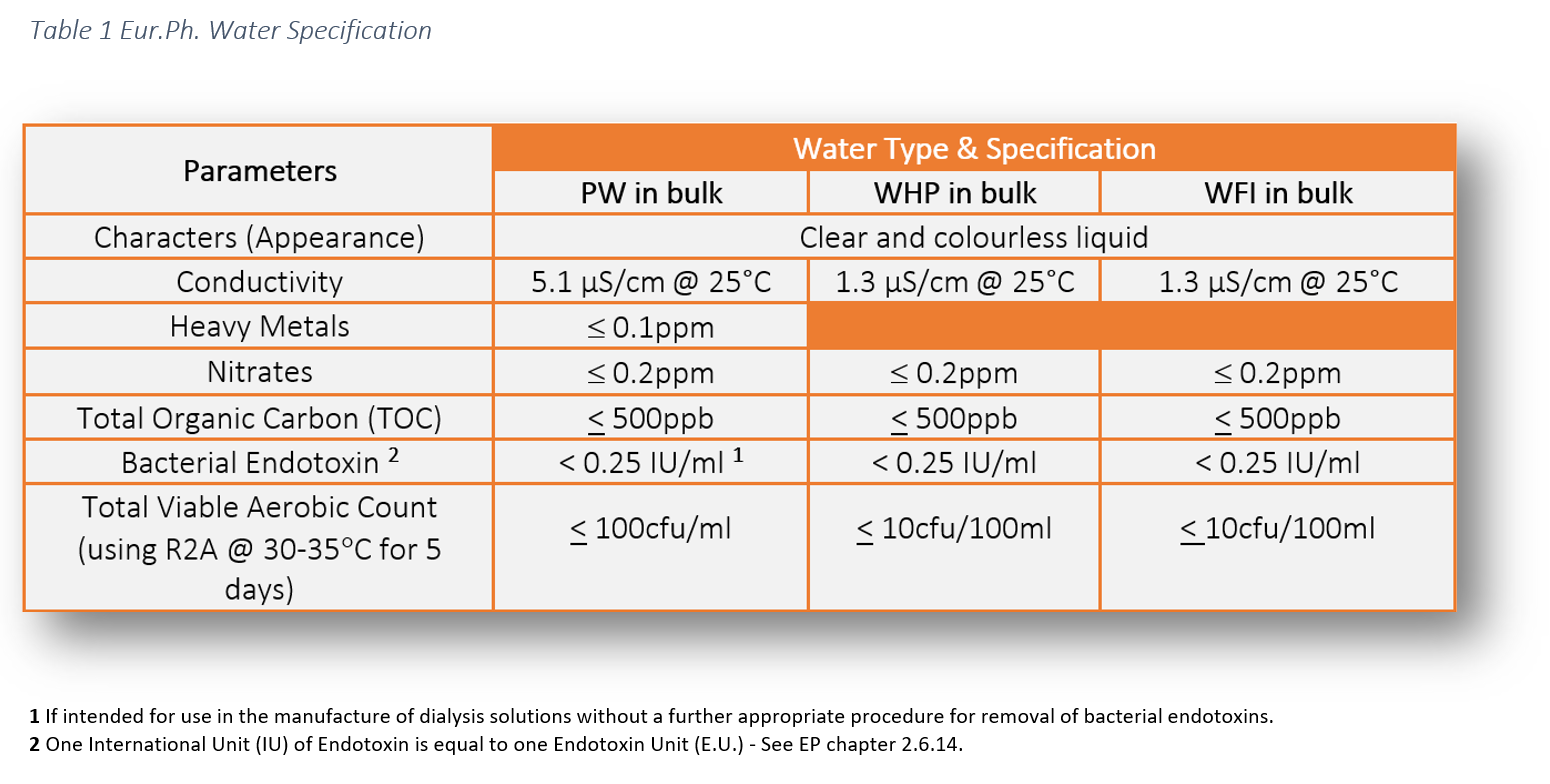
The legal requirement to comply with pharmaceutical water specifications is defined in the pharmacopoeial monographs. These water specifications can be found in the following pharmacopoeias: USP, Ph Eur and JP. This literature establishes the minimum quality standards within the industry which are enforceable by the regulators. As manufacturers and services providers we have a responsibility to ensure the highest level of operational control to ensure product safety for patients.

How can we help assure compliance?
At Honeyman we are able to provide the full suite of microbiological and chemical testing to ensure compliance with the following:
- European Pharmacopoeia (Ph. Eur.)
- In-house specifications
The chemical water quality of WHP is comparable with PW, however, the difference lies in the endotoxin specification.
To satisfy the cGMP microbiological and endotoxin quality component we are able to perform:
- Total viable count (TVC) via membrane filtration
- Limulus Amebocyte Lysate (LAL) turbidometric kinetic assay for the detection of endotoxins, also known as an Endotoxin test
Our suite of chemical tests to ensure compliance include:
- Conductivity
- pH
- Nitrates
- Total Organic Carbon
- Heavy Metals
Our Customers: