Endotoxin Testing
For water, finished product and raw materials
What is Endotoxin Testing?
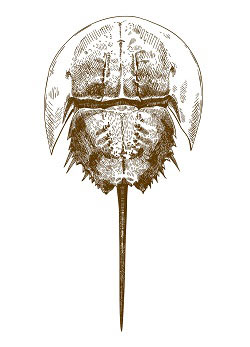
Endotoxins are components of the gram-negative bacterial cell wall released upon disruption of the cell wall. Limulus Amebocyte Lysate (LAL) turbidometric kinetic assay uses a particular enzyme from the Horseshoe Crab to detect endotoxin. Upon contact with endotoxin, the LAL enzyme causes clotting which can be measured with photo-spectrometry based on solution turbidity.
Why do we do it?
Endotoxin levels are controlled and monitored as they can elicit a pyrogenic response when introduced to the bloodstream. All Pharmacopoeias and regulatory bodies outline the submission and maintenance of pyrogen and endotoxin testing for regulated pharmaceutical products. It is the responsibility of the manufacturing company to define a sampling plan based on the complexities of the manufacturing process and endotoxin removal steps.
How can we help assure compliance?
Honeyman Group is able to perform cGMP endotoxin analysis on a wide range of raw materials, API’s and finished products. We are experts in the validation of endotoxin recovery after developing methods for an array of product presentations and formulations. Validation is essential as certain products can interfere with the LAL assay via enhancement or inhibition. We employ a highly sensitive assay method which allows us to detect as low as 0.001 EU/ml.
Once these parameters have been established during the validation we are able to perform routine Endotoxin analysis via the following method:
- Limulus Amebocyte Lysate (LAL) turbidometric kinetic assay
Bacterial Endotoxin Testing
To Request a Quotation or Book a Test Please Contact Us:
Our Customers: